No.3597
Cancer-Focused Genetic Testing Market in Japan: Key Research Findings 2024
Size of Cancer-Focused Genetic Testing Market in Japan for FY2023 Reached 13,700 Million Yen, 110.5% of Previous Fiscal Year
Yano Research Institute (the President, Takashi Mizukoshi) has surveyed the domestic cancer-focused genetic testing market and found out the trend of in vitro diagnostic testing agents and equipment, business development by market players, and future directions.
Market Overview
Genetic testing can be classified into three major categories: Testing of pathogen genes, genetic testing of human somatic cells, and genetic testing in which the changes are genetically inherited. In genetic testing of human somatic cells, advances in genetic analysis have enabled identification of gene alterations in a cancer cell. Cancer genomics or cancer genomic medicine has attracted attention, as improvement of treatment efficacy and reduction in side effects risks are expected through detailed analysis of gene information in a cancer cell and by choosing highly effective agents to the specified gene alterations.
In genetic testing for cancer, there are a companion diagnostic test that finds out optimal treatment for each patient by detecting specific gene mutations within a cancer cell and a cancer genomic profiling test (oncology panel test) that comprehensively analyzes a volume of genes included in a cancer cell. The latter studies cancer-related alterations in numerous genes at once and explores the next drug therapy for patients who have no standard treatment or those who have finished it.
As a part of its cancer control measures, the government offers research subsidies and has eased the regulations. The Basic Plan to Promote Cancer Control Programs (Phase 4) emphasizes on promoting genomic medicine by swiftly establishing the medical system to provide it, boosting the growth of cancer-driver genetic testing market. Nevertheless, there still are many challenges to overcome, such as protecting personal testing data, ethical issues, and costliness.
Noteworthy Topics
Wider Acceptance of Multiplex Companion Diagnostic Agents/Devices
A companion diagnostic test is a genetic test to seek the optimal medicines for each of cancer patients by predicting treatment efficacy and side effect risks through examinations of genes and proteins within cancer cells.
Conventionally, one companion diagnostic agent has been used for a single gene mutation or protein, but the recently launched multiplex companion diagnostic system allows detecting of multiple gene alterations in a single test. This has led the medical fee system to recommend simultaneous testing for multiple items.
Especially in the realm of lung cancer, market launch of a companion diagnostic system for identifying mutations in four driver genes (EGFR, ALK, BRAF, and ROS1) that cause non-small cell lung cancer in a single test has triggered the shift from individual gene alteration tests to multiplex diagnostic tests. In addition to this diagnostic system having expanded the detectible gene mutation types, multiple clinical tester manufacturers have started developing similar multiplex diagnostic systems, boosting the market expansion.
Future Outlook
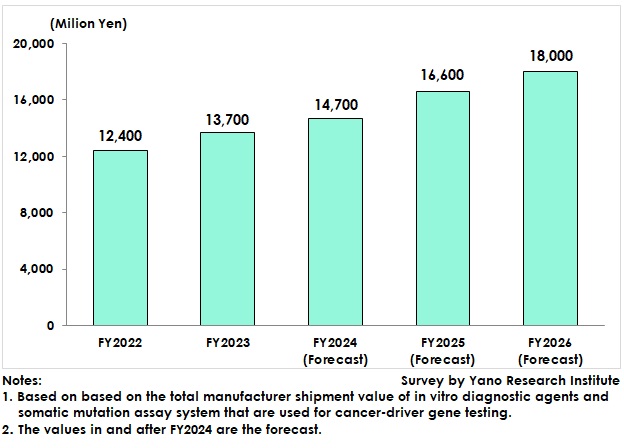
The market size of domestic cancer-focused genetic testing for FY2023, based on the shipment value at manufacturers, has been estimated at 13,700 million yen, 110.5% of previous fiscal year. The market is projected to value 14,700 million yen for FY2024, 107.3% of preceding fiscal year. Testing of cancer driver genes has conventionally been centered on companion diagnostics, but insurance coverage of genomic profiling tests for cancer in 2019 has stimulated the market growth.
In association with rising social expectations, the environment to offer cancer genomic medicine is about to take shape as base hospitals for the medicine being designated nationwide, in which cancer genomic profiling tests are available. Though the growth rate has slowed down, the number of tests conducted is on the rise. As a part of the national policy to promote cancer genomic profiling tests, relaxation of requirements to hold an expert panel (specialist team) review meeting and simplification of expert panels are discussed, which is likely for cancer genomic profiling tests to increase furthermore.
As a result of comprehensive knowledge of cancer genomics having been cataloged, genetic alterations have been discovered across multiple cancer types, which has led molecular targeted agents being effective for cancer types other than those indicated. Cancer genomic medicine is shifting from the treatment by organ to that by the type of genetic mutation and is entering the next stage.
Research Outline
2.Research Object: Clinical examination equipment/reagent manufacturers
3.Research Methogology: Face-to-face interviews by expert researchers (including online), indirect interviews, and literature research
Cancer-Focused Genetic Testing Market
Genetic testing can be classified into three major categories: testing of pathogen genes, genetic testing of human somatic cells, and genetic testing in which the alterations are genetically inherited. The genetic testing of human somatic cells is the testing to detect genetic structural irregularity that is unique to cancer cells as well as the testing for the gene expression profiling, which enable to clarify temporary information on genes of disease lesion or tissue that may mutate according to progress of the disease.
In this research the cancer-focused genetic testing market size has been calculated based on the total manufacturer shipment value of in vitro diagnostic agents and somatic mutation assay system that are used for cancer-driver gene testing.
<Products and Services in the Market>
Cancer companion test, cancer genomic profiling test
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.