No.2701
COVID-19 PCR Test Reagent and Equipment Market in Japan: Key Research Findings 2021
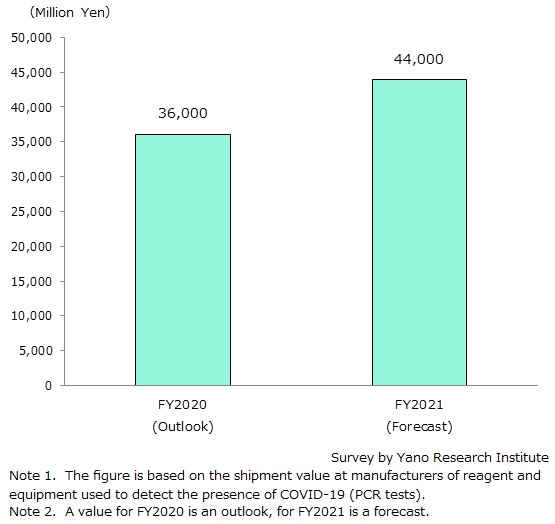
COVID-19 PCR Test Reagent and Equipment Market in Japan for FY2020 Expected to Reach 36,000 Million Yen
Yano Research Institute (the President, Takashi Mizukoshi) has carried out a research on the genetic testing market in Japan, and found out the trend of extracorporeal diagnostic medicines and medical equipment that are related to COVID-19 tests, the business operation of market players, and the future directions.
Market Overview
The market size of reagent and equipment related to COVID-19 PCR testing is expected to generate 36,000 million yen for FY2020, based on the shipment value at manufacturers.
During the global spread of COVID-19, Japan confirmed its first case of disease in January 2020; since then, the country entered first to third wave of COVID-19 infections, which led the government to declare a national state of emergency twice. As a precautionary measure to safeguard against COVID-19, the Japanese government is expanding PCR and antigen testing capacities, while test material manufacturers develop reagents and detection equipment for COVID-19-related genetic tests.
Noteworthy Topics
Special Demand Led by COVID-19 Outbreak
To bolster private testing centers such as medical institutions and clinical testing centers, to accelerate development of testing reagent, and to provide incentives to medical institutions that underpin social security system, the medical remuneration points allocated for COVID-19 PCR tests is extraordinarily high compared to those for genetic tests related to other infectious diseases. In addition to the acceleration of pharmaceutical approval review, an exceptional arrangement is made to authorize application of medical remuneration not only for extracorporeal diagnostic medicines but also for reagents that are yet to obtain pharmaceutical approval.
Against the backdrop of subsidies for reagents and equipment for PCR tests, installation of genetic testing equipment increased at medical institutions and regional medical testing centers. Active attempts are underway to expand testing programs to distinguish the novel coronavirus carriers flexibly and smoothly.
Future Outlook
As a recent trend, circulation of new strains of COVID-19 has been reported from all around the world. While the infections of new variant are confirmed in Japan, a fourth and fifth wave of coronavirus infections are much feared, and future remains uncertain. With reinforcement of surveillance system for new strains of COVID-19 and development of new reagents underway, it is assumed that development of new testing methods will make progress at many test material makers. Thus, demand is expected to rise for the coronavirus tests for both original strain and for new strains.
The market size of reagent and equipment related to COVID-19 PCR tests is projected to generate 44,000 million yen for FY2021 (122.2% of the previous fiscal year).
Research Outline
2.Research Object: Manufacturers of equipment and reagent used for clinical test
3.Research Methogology: Face-to-face interviews by the expert researchers, survey by telephone/email, and literature research
Genetic Testing
The genetic testing can be categorized into three main types: (1) Genetic identification of pathogens that looks for the viruses' genetic material, (2) genetic testing on somatic cells, and (3) genetic test to determine a person’s chance of developing or passing on a genetic disorder.
In this research, the market size is calculated for reagent and equipment used to detect the presence of COVID-19 (PCR test). The market includes genetic test kits and fees for voluntary tests (test fees not covered by insurance).
Nonetheless, reagent and equipment for antigen testing and antibody testing are not included.
<Products and Services in the Market>
Reagent and equipment used for COVID-19 PCR tests
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.