No.2226
Active Pharmaceutical Ingredient & Intermediate Market in Japan: Key Research Findings 2019
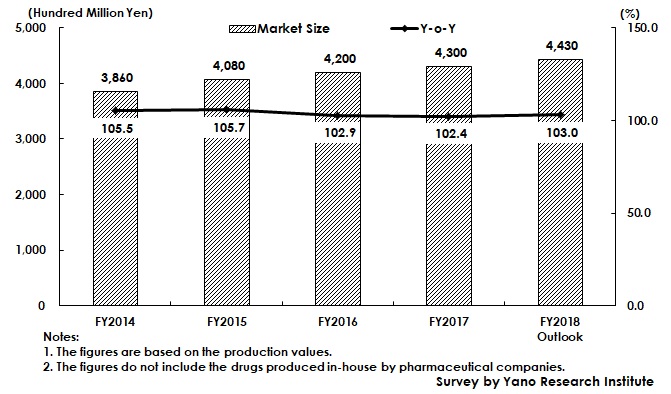
Active Pharmaceutical Ingredient & Intermediate Market in FY2018 Projected to Increase by 3.0% to Achieve 443 Billion Yen
Yano Research Institute (the President, Takashi Mizukoshi) carried out a survey on the domestic active pharmaceutical ingredient and intermediate market, and found out the market trends, trends of market players, and future outlook.
Market Overview
The domestic production value of drugs in 2018, excluding those imported, is estimated to have remained somewhat flat at 6,840,000 million yen (quoted from Statistics of Production by Pharmaceutical Industry by Ministry of Health, Labour and Welfare), while the market size of bulk drugs and intermediate for pharmaceuticals (based on the production value excluding those produced in-house by pharmaceutical companies) is expected to have achieved 443,000 million yen, attaining 3.5% of CAGR (Compound Annual Growth Rate) between FY2014 and FY2018.
The factors such as NHI price revision, patent expirations in major drugs, promotion to use generic drugs, and decreasing number of new drugs being approved, have affected the domestic production value of entire drugs (excluding imported products) to go through ups and downs in recent years. On the other hand, the domestic market of bulk drugs and intermediate for pharmaceuticals has continued growing.
Many pharmaceutical companies have propelled to outsource manufacturing, aiming to improve efficiency of company operations, and eventually to increase profits. Such increase of outsourcing at pharmaceutical companies has expanded the market of bulk drugs and intermediate for pharmaceuticals, which increased the number of market players such as from the chemistry industry including some major comprehensive chemistry enterprises, and some companies from overseas, in addition to some existing companies that have long dedicated to the market.
Noteworthy Topics
Tighter Quality Control Required
As the market expands, there has been growing demand for quality control such as reaching the GMP (Good Manufacturing Practice) standard. Reaching a certain quality is not a mere request, but rather, has become a basic prerequisite for a commissioned manufacturer to receive orders from the customers. In addition, as the market has become global, the level of quality control needs to be the same level as FDA (Food and Drug Administration). While conforming to strict quality control, the commissioned manufacturers are required to respond to various factors, including prices, delivery period, stable supply capacity, technological capabilities, etc. The prices have always been requested to be reduced, while recently, the customers have started focusing on the delivery speed: They require shorter initial phases of product development, requesting the delivery of active ingredients and intermediate within shorter time period. Therefore, while the suppliers of active ingredients and intermediate are required to cope with cost reduction, they are also required to enhance quality control as well as to achieve faster delivery.
In addition, continuous capital investment is necessary. In order to be able to continuously accept the orders from the customers (pharmaceutical companies), it is significant to appeal them by introducing the latest equipment as possible. Also, it is important to be able to respond to the changing customer needs, such as a shift from a large item to various small items. In the case of producing small volume of products, because large-scale investment to facilities is not appropriate or affordable, an optimal facility according to production volume is required. As for manufacturing highly potent API, a facility to solely deal with it is needed.
Future Outlook
In order to forecast the future market of active ingredients and intermediate for pharmaceuticals, both the growth factors and obstacles are needed to be considered. There are following growth factors in the market: Propelled outsourcing of manufacturing drugs by pharmaceutical companies, restructuring of manufacturing facilities at pharmaceutical companies, propelled outsourcing for manufacturing highly potent API, promoted development of drugs with the molecular weight in the ranges between 1,000 and 10,000, production overseas by the domestic active ingredient and intermediate manufacturers, and stricter environmental regulations in China. On the other hand, the obstacles can be the following: Promoted use of generic drugs, decreasing number of new drugs being approved, decrease in major drugs that have been newly approved and released, expiration of major drugs patent overall done with, and emerge of overseas companies.
While outsourcing of manufacturing new drugs is to increase among major pharmaceutical companies, commissioned manufacturing are likely to accept more orders as they become more trusted by augmenting facilities and improving quality of the deliverables. Nevertheless, the market growth can slow down, due to decrease of long-term listed items stemming from propelled use of generic drugs and to NHI price revision. In addition, now that outsourcing of manufacturing has expanded to a certain degree, its growth rate can slow down, while ever-fiercer competition waiting among increasing number of market players.
Research Outline
2.Research Object: Companies commissioned to produce or distributors of active ingredients and intermediate for pharmaceuticals, and general pharmaceutical companies
3.Research Methogology: Face-to-face interviews by expert researchers, surveys via telephone/email, mailed questionnaire, and literature research
About Active Pharmaceutical Ingredients and Intermediate
Active pharmaceutical ingredients are materials of active ingredients that are needed in the process of producing pharmaceutical products. Intermediate is the material produced in the process of producing active ingredients, during which they go through such processes as molecular change and purification. The market of active pharmaceutical ingredients and intermediate in this research indicates the market of pharmaceutical ingredients and intermediate domestically produced, but excludes those produced in-house by pharmaceutical companies.
<Products and Services in the Market>
Active pharmaceutical ingredients and Intermediate
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.