No.3324
Pharmaceutical Contract Manufacturing Market in Japan: Key Research Findings 2023
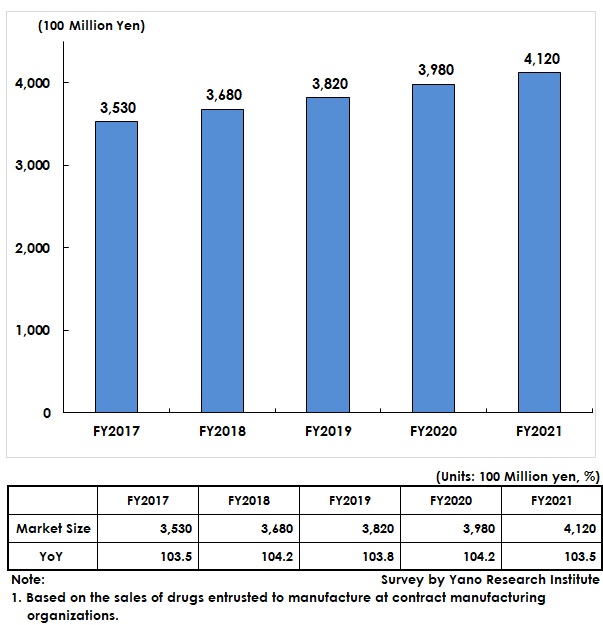
Estimated Pharmaceutical Contract Manufacturing Market Size Reached 412,000 Million Yen, Up by 3.5% from Previous Fiscal Year
Yano Research Institute (the President, Takashi Mizukoshi) has carried out a survey on the domestic pharmaceutical contract manufacturing market and found out the trend of contract manufacturing organizations and pharmaceutical companies, the market perspectives, and challenges.
Summary of Research Findings
After the Revised Pharmaceutical Affairs Law became effective in 2005, pharmaceutical companies without any factories have become allowed to be qualified and approved for selling the products they designed and developed, enabling them to fully outsource the manufacturing sector. In association with spread of generic drugs, joint development of generic drugs has expanded through alliance between generic drugs companies and pharmaceutical companies. With advancement of outsourced manufacturing of drugs, number of contracted cases at contract manufacturing organizations and production volume have continued expanding.
The pharmaceutical contract manufacturing market has been on the rise with its growth rate kept at 3 to 4 percent since FY2017. The market for FY2021 is estimated as 412,000 million yen, a rise by 3.5% from the previous fiscal year, because of steady growth at contract manufacturing stemming from increased joint development of generic drugs and decreasing production volume of long-term listed items due to declining drug prices.
Noteworthy Topics
Building of Quality Assurance Mechanism and Enhancement in Quality Management System
The Revised Pharmaceutical Affairs Law requires enhanced quality assurance even at contract manufacturing organizations, which has caused them to try building a quality assurance mechanism.
They also are needed to strengthen a quality management system: Not only are they required to pass the GMP (Good Manufacturing Practice) standards, but will no doubt be required to have a quality management system and abilities that meet the further-stricter FDA (Food and Drug Administration) level if the legislation conforms to global standards. Therefore, they urgently need to improve the quality management abilities.
Proactive capital investment for deployment of automated manufacturing systems at each contract manufacturing organization advances the automation of manufacturing processes, which makes the entire operations easy. Because such a situation requires training and securing of manpower (software) to act flexibly, rather than machine (hardware) performance, many companies tend to increase manpower.
Furthermore, responding to revised ministerial ordinance on GMP defines the future, too. This ordinance, that has gone through large-scale revision for the first time in 16 years, requires consistency with the GMP guidelines by Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S), i.e., the substantial international standard. Therefore, there observed are many companies reviewing their in-house quality management systems.
In recent years, there have been many accidents and incidents at drug manufacturing sites that cannot help doubting about the safety. For manufacturing contract organizations, the establishment of a GMP organizational structure and operational system that includes the business management and directors liable to ensure the establishment and maintenance of a controlled state is a major issue in the future pharmaceutical contract manufacturing market.
Research Outline
2.Research Object: Companies related to pharmaceutical contract manufacturing
3.Research Methogology: Face-to-face interviews (including online) by expert researchers, and literature research
Pharmaceutical Contract Manufacturing Market
Pharmaceutical contract manufacturing in this research is defined as the business where pharmaceutical companies outsource all or a part of manufacturing processes of their pharmaceutical products that they designed and developed (or jointly developed with other companies).
There are two kinds of contract manufacturing of pharmaceutical products: 1) outsourcing of manufacturing processes to outright external firms and 2) outsourcing manufacturing processes to affiliated companies. This research has excluded outsourcing to affiliated companies. Note that the affiliated enterprises mentioned above are those subsidiaries that manufacture only for their parent companies. If a company is a wholly owned subsidiary of a pharmaceutical company but performs contract manufacturing not only for its parent but also for other companies, it is then defined as an external enterprise and has been included in the market size.
The market size has been calculated based on the sales of drugs entrusted manufacture at contract manufacturing organizations.
<Products and Services in the Market>
Pharmaceutical Contract Manufacturing
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.