Pharmaceutical Contract Manufacturing Market: Key Research Findings 2015
Research Outline
- Research period: April to June, 2015
- Research target: Pharmaceutical contract manufacturing companies and consignors
- Research methodologies: Face-to-face interviews by the specialized researchers, mail-in questionnaire, and literature research
<What is Pharmaceutical Contract Manufacturing?>
Pharmaceutical contract manufacturing in this research is defined as the business where large pharmaceutical companies outsource all or a part of manufacturing processes of their pharmaceutical products in the wake of their development and designing of such products (including joint development with other companies). There are two kinds of contract manufacturing of pharmaceutical products: 1) outsourcing of manufacturing processes to outright external firms and 2) outsourcing manufacturing processes to affiliated companies. This research excludes outsourcing to affiliated companies.
Note that the affiliated enterprises mentioned above are those subsidiaries manufacturing only for their parent companies. Even if a company is a wholly owned subsidiary of a pharmaceutical company, but performs contract manufacturing not only for its parent but also for other companies, it is then defined as an external enterprise.
<What is the Pharmaceutical Contract Manufacturing Market?>
The pharmaceutical contract manufacturing market in this research is based on the sales of contract manufacturing at pharmaceutical contract manufacturing enterprises.
Summary of Research Findings
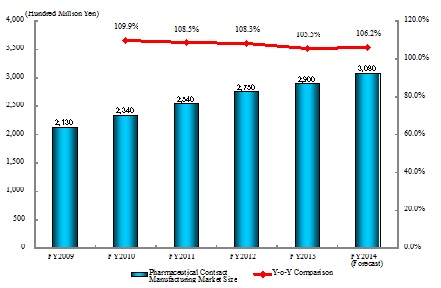
- Pharmaceutical Contract Manufacturing Market in FY2013 Rose by 5.5% to Attain 290 Billion Yen on Y-o-Y Basis
Size of the pharmaceutical contract manufacturing market in FY2013 is estimated as 290 billion yen, a rise by 5.5% from the previous fiscal year. The market has been influenced by both expansion of generic drugs market that led to increasing number of orders for manufacturing drugs including those jointly developed, and by the shrinking market of patent-expired original medicines that brought about decreasing orders for manufacturing specific original drugs. Although the contracts for manufacturing original medicines may decrease, the pharmaceutical contract manufacturing market is expected to continuously stay stable due to prospected growth of contract manufacturing for newly patent-expiring drugs and to expansion of the generic drugs market.
- Pharmaceutical Contract Manufacturers Aim to Add Value by Shifting From CMO to CDMO through Facility Augmentation, Enhancement of Product-Quality Management, and of Pharmaceutical Development
Driven by the recent increase of needs and prospective market expansion, the pharmaceutical contract manufacturers are trying to respond to the consignor needs and acquire more orders by augmenting facilities and strengthening product quality management systems. Many of such market players are pressing ahead with enhancement of pharmaceutical development sector, aiming to shift from being CMO (Contract Manufacturing Organization) to CDMO (Contract Development & Manufacturing Organization), which enables them to provide not only manufacturing of the conventional commercial medicines but also developing of the investigational new drugs. The contract manufacturers are considered to be trying to make themselves higher valued companies by being able to provide one-stop integrated services from developing investigational drugs to manufacturing medicines whenever they receive orders.
- Figure 1: Transition of Pharmaceutical Contract Manufacturing Market Size