No.2469
Personalized Medicine Market in Japan: Key Research Findings 2020
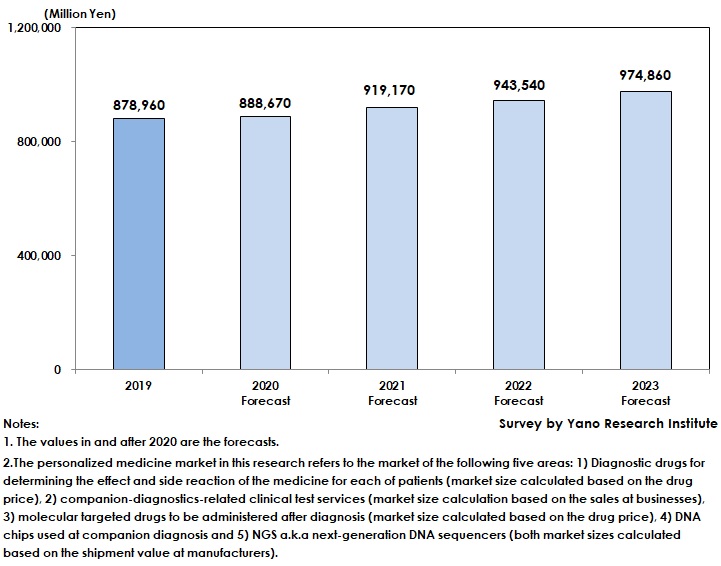
Domestic Personal Medicine Market Expected to Generate 878,960 Million Yen
Yano Research Institute (the President, Takashi Mizukoshi) carried out a survey on the domestic personalized medicine market and found out the trends by each market category, business development by market players, and future perspectives.
Market Overview
To take account of genetic difference by each patient, personalized medicine that creates optimal treatment plans for each of the patients has been attracting attention. This research targets the following five areas: 1) Diagnostic drugs for determining effectiveness and side reaction of the medicine for each of patients, 2) companion-diagnostics related clinical test services, 3) molecular targeted drugs that are administered after diagnosis, and 4) DNA chips as well as 5) NGS (next-generation DNA sequencers).
The domestic personal medicine market in 2019 was estimated as 878,960 million yen. Due to extensive use of molecular targeted drugs and prospering development of diagnostic drugs, the market has been on the rise. In addition, clinical genome sequencing that utilizes NGS has been in practical use.
Noteworthy Topics
Multiplex Testing Fully in Practice for Simultaneously Inspect Multiple Genetic Mutations for Potential Cancers
Companion diagnostics basically use one diagnostic drug for one symptom of gene mutation. In recent years, however, some diagnostic drugs available to test multiple symptoms have started being developed and launched. In Japan, a reagent that can detect KRAS and NRAS mutations (RAS gene mutations) for colorectal cancer was released in 2015. In 2019, a multiplex companion diagnostic system available to simultaneously detect 4 types of driver genes relating with non-small cell lung cancer was approved to be covered by insurance.
In addition to cancer genome medicine having been pressed ahead as the national policy, development of genetic panel inspections for cancer is also in progress, encouraging social implementation of clinical sequences using NGS. In Japan, two types of cancer genetic panel systems have been listed for coverage under national health insurance since 2019. Such moves of driving practical implementation of clinical sequence using NGS have attracted expectation from gene-related companies and doctors.
Future Outlook
As some medical institutions were forced to suspend medical care for outpatients in 2020 due to COVID-19 pandemics, the demand for testing decreased and slowed down the market growth. The personal medicine market in 2020 is forecasted to attain 888,670 million yen, a rise by 1.1% from the previous year.
Nevertheless, the personal medicine market is projected to expand furthermore to achieve 974,860 million yen by 2023, driven by molecular targeted drugs and diagnostic drugs.
Research Outline
2.Research Object: Pharmaceutical enterprises, manufacturers of clinical test/diagnosis apparatuses, other related companies
3.Research Methogology: Face-to-face interviews by our expert researchers, and indirect surveys
The Personalized Medicine Market
The personalized medicine market in this research refers to the market of the following five areas: 1) diagnostic drugs for determining the effect and side reaction of the medicine for each of patients (market size calculated based on the drug price), 2) clinical test services related with companion diagnostics (market size calculation based on the sales at businesses), 3) molecular targeted drugs to be administered after diagnosis (market size calculated based on the drug price), 4) DNA chips and 5) NGS a.k.a next-generation DNA sequencers (market size calculated based on the shipment value at manufacturers), with DNA chips used at companion diagnosis and NGS expected to expand the use at clinical sites.
<Products and Services in the Market>
Molecular targeted drugs, diagnostic drugs for determining the effect and side reaction of the medicine, clinical test services related with companion diagnostics, DNA chips and NGS (next-generation DNA sequencers)
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.