No.2589
AI-Assisted Diagnosis/Medical Treatment Systems Market in Japan: Key Research Findings 2020
Domestic AI Systems for Assisting Diagnoses and Treatment Expected to Expand after 2020
Yano Research Institute (the President, Takashi Mizukoshi) carried out a survey on the domestic MedTech trends and found out the market penetration of related medical equipment, business development by market players, and future perspectives. Disclosed here is the market forecast on AI systems for assisting diagnoses and treatment.
Market Overview
MedTech is a coined word from medical technology and is the act of providing new values, or the medical products and services that provide new values, by using state-of-the-art technologies such as AI, IoT, XR (VR [virtual reality], AR [augmented reality], MR [mixed reality]), 5G, 4K/8K when providing medical care, diagnoses and treatment.
In the medical sphere, there have been some attempts to provide new values in the products and services by utilizing the latest ICT technologies for assisting medical care, diagnoses and treatment. In recent years, AI-embedded diagnosis assistant systems, 8K endoscopic surgical systems, surgical operation assistance services utilizing XR, etc. have been released.
Among such technologies, the development of AI has been positioned as a national policy and AI implementation in the society has been in progress, which includes AI-equipped medical instruments released by multiple manufacturers and actually used at some medical institutions for assistance of diagnoses and treatment.
In recent years, there are some attempts in medical practices of utilizing XR technologies (VR, AR, MR) that express and enable experiencing unreal world. The applications of XR-used medical instruments are expanding to medical educations and training, assistance of medical care, surgical operations, and rehabilitations. They are expected to improve the educational quality and efficiency, to increase accuracy in surgical operations, and to quantify the diagnoses and medical treatments that used to be qualitative.
Noteworthy Topics
Digital Technologies Becoming Essential in Medical Practices and Require Innovation
AI and other digital technologies are indispensable in medical practices for the future and are expected to transform the existing medical care. Utilization and social implementation of AI technologies have been positioned as a significant strategy in the Japanese government, which is driving some attempts of preparing data platforms and of data utilizations for contribution of AI development. Diagnoses assistance using medical images have drawn a particular attention as an example of AI in the medical sphere.
In Japan, as a result of various studies and demonstrations carried out until 2018, an AI system for assisting diagnoses of endoscopic images became the first AI-embedded medical equipment to have acquired pharmaceutical approval in December 2018. By 2019, an AI system for diagnoses assistance of a cerebral aneurysm has also earned pharmaceutical approval. In 2020, as a measure against COVID-19 infections, MHLW (Ministry of Health, Labour and Welfare) announced preferential and speedy procedure for necessary pharmaceutical approvals, which led to release two AI systems for assisting diagnoses of COVID-19-caused pneumonia.
There are increasing numbers of companies constructing medical platforms to list AI products and services, accelerating the attempts of social implementation of AI technologies. Because data is essential for those medical equipment using digital technologies, preparation of digital bases such as construction of medical public databases and of digital platforms has been simultaneously in progress as a national policy.
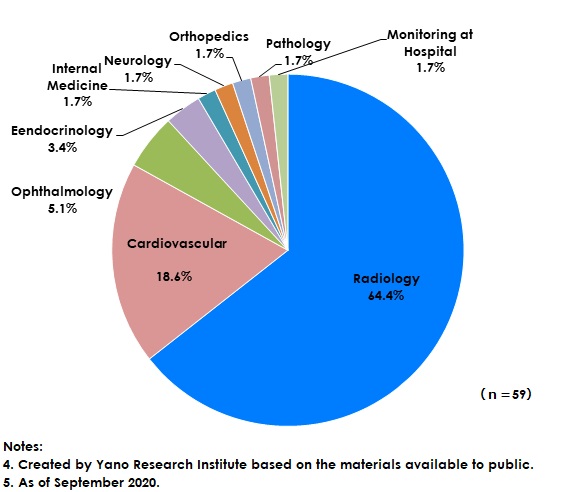
On the other hand, the U.S. has also been eager for development and implementation of AI year by year, and accelerating such activities after 2018. As of September 2020, 59 items of products are confirmed to have been certified as AI-embedded medical equipment by FDA (Food and Drug Administration). When categorizing these by medical sector, 38 items belong to radiology, occupying 64.4% of the entire items, followed by 11 items belonging to cardiovascular segment which account for 18.6%. There are 3 items from ophthalmology, accounting for 5.1%. The functions of AI-embedded diagnoses-assistance systems have diversified, which not only contribute in supporting of detection and diagnoses but also in decision-making assistance for triage or medical practices.
Future Outlook
Both AI systems for diagnoses assistance and for medical-treatment assistance are considered to be in the dawning era, and the government has been promoting the development and utilization of AI technologies in medical areas in the opportunities such as the “Future Investment Conference” and “Artificial Intelligence Strategical Meeting.” In the “Conference to Promote Utilization of AI in Medical Treatment and Health” led by MHLW, six areas including image diagnoses and medical-treatment assistance have been specifically chosen for AI development and implementations, accelerating such development and implementations furthermore. The phase of AI development seems to be shifting from verification (including “studies” and “demonstrations”) to utilization of AI products by 2020.
Expansion of COVID-19 infections drove the society to introduce new normal, some of which are “not to touch things” and “not to contact,” and increased the use of online medical care and the early approval of AI systems for diagnosing COVID-19-caused pneumonia, which socially draw attention. AI products and services in the medical sphere are likely to increasingly used at medical institutions as one of the troubleshooting solutions.
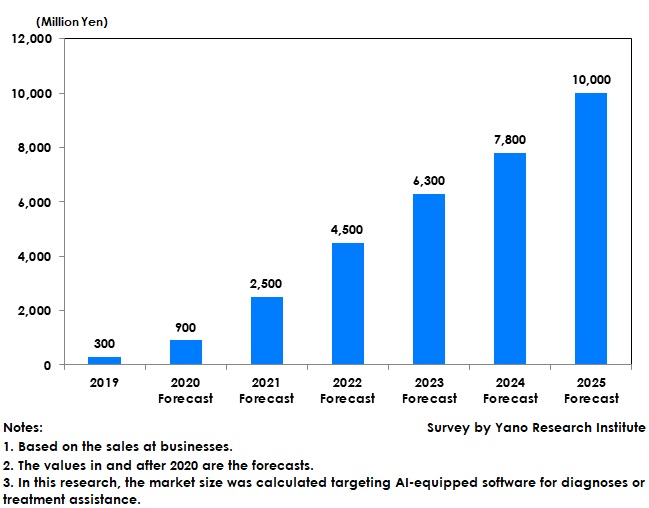
The government has been pressing ahead with building of medical databases and utilization of medical-treatment data for accelerating AI development. The companies in the market are also driving their R&D aiming at releasing their products in around 2022 to 2024, which is likely to increase the number of AI-embedded medical equipment and to diversify AI applications for the future. Furthermore, building of medical platforms is on the way by major modality manufacturers to promote using of diversifying AI applications. As AI-implemented systems for assisting diagnoses as well as medical treatment are expected to permeate in medical institutions, the market size of AI systems for diagnoses/treatment assistance is forecasted to expand to 10 billion yen based on the sales at businesses.
Research Outline
2.Research Object: Medical equipment manufacturers, medical IT companies, pharmaceutical companies, and other related companies
3.Research Methogology: Face-to-face interviews by the expert researchers, surveys via telephone & email, and literature research
What is MedTech?
MedTech is a coined word from medical technology and is the act of providing new values, or the medical products and services that provide new values, by using state-of-the-art technologies such as AI, IoT, XR (VR [virtual reality], AR [augmented reality], MR [mixed reality]), 5G, 4K/8K when providing medical care, diagnoses and treatment. AI and other digital technologies are indispensable in medical practices for the future and are expected to transform the existing medical care.
In this research, the market size was calculated targeting AI-equipped software for diagnosis or treatment assistance in the process of social implementation in recent years.
<Products and Services in the Market>
AI-equipped software for diagnosis assistance or treatment assistance, Medical equipment utilizing XR (VR, AR, MR), medical platform
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.