No.2003
Market of Bulk Drugs and Intermediate for Pharmaceuticals in Japan: Key Research Findings 2018
Market of Bulk Drugs and Intermediate for Pharmaceuticals in Japan: Key Research Findings 2018
Yano Research Institute (the President, Takashi Mizukoshi) has conducted a survey on the domestic market of bulk drugs and intermediate for pharmaceuticals and has found out the market trends, trends of market players, and future outlook.
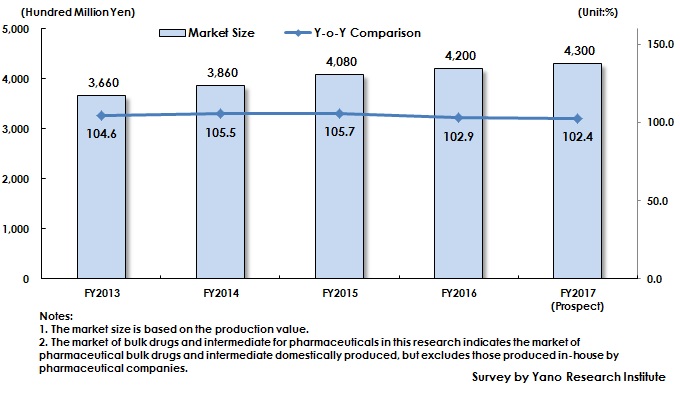
Market Overview
While estimated domestic production value of drugs in 2017, excluding those imported, has been on the decline to reach 6,200,000 million yen (quoted from Statistics of Production by Pharmaceutical Industry by Ministry of Health, Labour and Welfare), the market size of bulk drugs and intermediate for pharmaceuticals (based on the production value excluding those produced in-house by pharmaceutical companies) is expected to achieve 430,000 million yen, attaining 4.1% of CAGR (Compound Annual Growth Rate) from FY2013 to FY2017.
The factors such as NHI price revision, promotion to use generic drugs, and price reduction of long-term listed items have affected the domestic production value of entire drugs (excluding imported products) to slow down and then to turn to negative growth. On the other hand, the domestic market of bulk drugs and intermediate for pharmaceuticals has continued growing.
Many pharmaceutical companies have been promoting outsourcing especially of manufacturing aiming to improve profit by such a measure to improve work efficiency. This trend has expanded the market of bulk drugs and intermediate for pharmaceuticals, but the growth rate has been slowing down since FY2016, affected by shrinking domestic production value of drugs (excluding imported products).
Noteworthy Topics
In spite of favorable environment for the domestic market of bulk drugs and intermediate for pharmaceuticals, the market players are facing many challenges and problems for them to continue growing. The largest issue in recent years is to respond to ever-demanding customers who request the companies of bulk drugs and intermediate for pharmaceuticals to be adaptable in terms of both quality and price simultaneously. The customers, i.e., pharmaceutical companies, had been demanding also in the past, but because of the specific characteristics of the products, they used to have higher consciousness in the quality.
However, enhanced suppression of medical fees, decreasing number of new drugs approved and of new blockbuster drugs, changes in the environment surrounding pharmaceutical companies such as lapse of the patent of their major products, together with revised Pharmaceutical Affairs Act in 2005 allowing overseas companies such as India, China, and South Korea to enter the Japanese market with their low-price drugs, have all changed the idea of pharmaceutical companies to consider more about the balance between product quality and price.
Research Outline
2.Research Object: Companies that domestically develop business of producing/selling bulk drugs and intermediate for pharmaceuticals, and general pharmaceutical companies
3.Research Methogology: Face-to-face interviews by the expert researchers, surveys via telephone/email, mail-in questionnaire, and literature research
What are Bulk Drugs and Intermediate for Pharmaceuticals?
Bulk drugs are materials of active ingredients that are needed in the process of producing pharmaceutical products. Intermediate is the material produced in the process of producing bulk drugs, during which they go through such processes as molecular change and purification. The market of bulk drugs and intermediate for pharmaceuticals in this research indicates the market of pharmaceutical bulk drugs and intermediate domestically produced, but excludes those produced in-house by pharmaceutical companies.
Published Report
Contact Us
The copyright and all other rights pertaining to this report belong to Yano Research Institute.
Please contact our PR team when quoting the report contents for the purpose other than media coverage.
Depending on the purpose of using our report, we may ask you to present your sentences for confirmation beforehand.